Cooperative activities between INCT-IBISAB with other institutions
⇒ DINTER – Inter-institucional PhD Course in Morphological Sciences between UFRJ and UFC
⇒ International Network for the Study of Infant Diarrhea and Malnutrition (MAL_ED)
⇒ International Program of Cooperation between UFC £ University of Virginia

The researchers’ commitment and the INCT-IBISAB research infrastructure were fundamental to the newly created inter-institutional PHD course in Morphological Sciences involving the Federal University of Rio de Janeiro (UFRJ) and the Federal University of Ceará (UFC), under the academic coordination of Prof. Vivaldo Moura Neto (UFRJ), Reinaldo Oriá and Gerly Brito (UFC). The scientific activities will be held at the Department of Morphology of UFC as well as in the IBISAB facilities.
Exploring the potential for nucleation of the Morphological Sciences Program of UFRJ, the new course seeks to promote training skills in morph-functional sciences in order to awaken new vocations to the academic life and trigger the formation new research groups interested in the morphological studies of the digestive and nervous systems in the UFC School of Medicine and in the INCT-IBISAB, thus improving the understanding and management of prevalent diseases among Semiarid populations. Furthermore, the partnership with the UFRJ will provide the full use of infrastructure already available in the INCT-IBISAB enabling teaching careers in Morphological Sciences in our Brazilian universities, especially those located in the University campi spread in the Semiarid countryside. This is the first postgraduate program (PhD level) in Morphological Sciences in the Brazilian North and Northeast regions that, certainly, will contribute to minimizing regional disparities.

This network (RECODISA) is formed by researchers of three Brazilian institutions, Federal University of Ceará (UFC), Ceará State University (UECE), University of Fortaleza (UNIFOR), and an international institution, the University of California, Davis (UC Davis, USA) with financial support from the Ministry of Science and Technology (FINEP Cross Order, Brazil), having as main objective the identification of specific etiology and development of immune compounds in the milk of transgenic goats for the prevention and treatment of childhood diarrhea in the semiarid areas (for details see www.recodisa.ufc.br).
Motivated by the idea of offering immune compounds commonly found in human milk in the milk of animals through the use of biotechnology and, then to benefit the health of children at risk in the population of the semi-arid region of Brazil, RECODISA participate in the five post-graduate courses: (a) Northeast Biotechnology Network - RENORBIO (Level in Capes scale: 5 (ranging from 3 to 7); (b) Pharmacology - UFC (level 6); (c) Medical Sciences - UFC (level 5); (d) Biotechnology (level 4; located in Sobral, a countryside city); and (e) the newly created inter-institutional PhD course in Morphological Sciences between UFC and UFRJ (level 6).

The impacts in terms of knowledge and technology transfer from INCT-IBISAB should be significantly amplified by RECODISA. The specific objectives of RECODISA are: (a) to determine the etiology of diarrhea in children 2-36 months of age seen at health facilities and to compare their profile with healthy controls from the urban community in Fortaleza, CE, Brazil; (2) To develop molecular methods for rapid and sensitive diagnosis of the etiologic agents and to evaluate the genetic variation of virulence genes of the major causes of childhood diarrhea; (3) to evaluate by a cross-sectional study the etiology of diarrhea in children aged 2-36 months old in the semiarid region of Brazil; (4) To establish lines of heterozygous transgenic goats in Brazil for the human lysozyme gene (HLZ) from UC Davis; (5) Purification and proteomic analysis of HLZ expressed in the milk of transgenic goats, (6) Characterization of the in vitro efficacy of the goat milk from HLZ transgenic lines against human pathogens commonly found in Brazilian semiarid; (7) To conduct preclinical and clinical testing of purified HLZ from the milk of transgenic goats, (8) To generate heterozygous strains of transgenic goats with the gene of human lactoferrin (HLF), through animal cloning procedures for somatic cell nuclear transfer (SCNT); (9) Purification and proteomic analysis of HLF expressed in the milk of transgenic goats; (10) characterization and in vitro efficacy of goat milk of the transgenic lines HLF against human pathogens common to semiarid; (11) conduct pre-clinical tests and clinicians with HLF and purified from the milk of transgenic goats for the HLF; (12) To generate heterozygous strains of transgenic goats for peptide enriched with glutamine and arginine (PGA) by means of animal cloning by SCNT; (13) Purification and proteomic analysis of PGA expressed in the milk of transgenic goats; (14) characterization of in vitro efficacy of goat milk of the PGA transgenic lines against human pathogens commonly found in Brazilian semiarid; (15) To conduct preclinical and clinical testing studies with purified PGA and with milk of transgenic goats for the PGA; (16) To create the breeding program for the establishment of homozygous lines of transgenic goats for the genes of HLZ, HLF and PGA; (17) characterization of colony-stimulating factor in human granulocytes (HG -CSF) expressed in the milk of transgenic goats; (18) Cloning of Transgenic females for colony-stimulating factor in human granulocyte (hG-CSF) by SCNT; (19) Purification and proteomic analysis of colony-stimulating factor in human granulocytes ( hG-CSF) expressed in the milk of transgenic goats, and, finally, (20) To conduct preclinical and clinical testing studies with purified hG-CSF.

In course of the first year we began the study on childhood diarrhea, covering eight cities located in semiarid with over fifty thousand inhabitants. Moreover, it has been developed two transgenic bio-products: goat milk with human lysozyme (HLZ) and goat milk with the colony-stimulating factor in human granulocytes. Such products are now undergoing tests in vitro and in vivo to demonstrate its feasibility and biological effectiveness for, then, to initiate the preclinical and clinical testing (Castro LA & Barros AK. Incentives for Brazilian health biotech. Nat Biotechnol . 27 (4) :317-8, 2009 and DR Brundige et al. Consumption of pasteurized human lysozyme transgenic goats' milk alters serum metabolite profile in young pigs. Transgenic Res 2009 Oct 22.). Our outlook for the second year is quite encouraging given the initial success in cloning these two byproducts, milk with HLZ (developed and produced at UC Davis) and milk with hG-CSF (developed and produced in State University of Ceara, UECE).

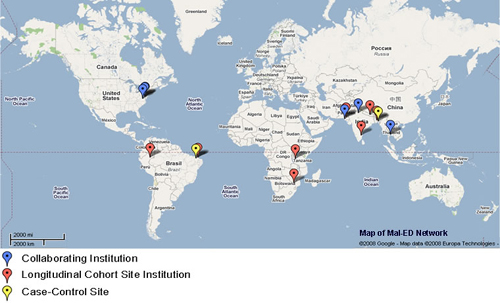
This network MAL_ED (Malnutrition_Enteric Diseases) was recently assembled upon the support of the Bill and Melinda Gates Foundation for the global study of malnutrition and infant diarrhea in the following countries: USA, Brazil, Bangladesh, South Africa, Tanzania, India, Pakistan, Nepal and Peru. Among the main organizers of the proposed international network, are the U.S., Bangladesh and Brazil, through this IBISAB (for details see the Internet www.upcibimed.ufc.br)
So far the INCT-IBISAB already developed and / or is developing several bio-markers and molecular probes for specific diagnosis of causative agents of infantile diarrhea, bio-markers for assessing intestinal inflammation, such as lactoferrin and others, as well as molecular probes, lactulose and mannitol, to determine the intestinal absorption area, permeability and intestinal damage, and are now IBISAB international reference for some of these products.

The project will establish a network of sites (multicenter) in developing countries in partnership with the United States for better understanding the risk factors for malnutrition, enteric diseases and their consequences for health, including the deficits in child development. After all, one in five children in developing countries is malnourished and marginal nutrition is associated with more than half of all deaths in children around the world. This project will carry out epidemiological studies (longitudinal and case-control) at multiple sites to assess the correlation level among: (a) infection by enteric pathogens, (2) intestinal barrier function changes, (3) immune response to vaccines, and (4) Malnutrition with deficits in child development.
Although some of these associations are already known, there are still few quantitative and qualitative data on the specific etiologic agents, incidence by age, co-infection status and further functional studies of the gastrointestinal tract with specific nutritional assessment - key data to establish the target for optimal interventions. Moreover, we will formulate models of geospatial distribution of risk factors for enteric diseases and malnutrition in order to extrapolate the findings of other studies involving our population. The multi-center network will also enable the standardization of resources, data and samples on a single platform for research, and use of new technologies, such as those to identify and characterize host biomarkers and intestinal microbiome related to nutritional status, disease susceptibility, and development. Defining risk factors and biomarkers of malnutrition may eventually be developed and evaluated to build optimal strategies for intervention and surveillance, initially at the MAL_ED network sites.
In brief, the MAL_ED network has three principal activities:
![]() Activity 1 To establish administrative structure to develop and manage a multi-center network in developing countries to study malnutrition and enteric infections.
Activity 1 To establish administrative structure to develop and manage a multi-center network in developing countries to study malnutrition and enteric infections.
![]() Activity 2 - To conduct longitudinal epidemiological study, prospective and case-control to identify factors associated with risk for enteric infections, chronic diarrhea, malnutrition, as well as impairment of bowel function, and response to vaccine development.
Activity 2 - To conduct longitudinal epidemiological study, prospective and case-control to identify factors associated with risk for enteric infections, chronic diarrhea, malnutrition, as well as impairment of bowel function, and response to vaccine development.
- To collect the phenotypic and environmental data centers, enabling integration of future studies from the expansion of collaboration in research and research activities translated and designed to reduce infant mortality.
![]() Activity 3 To develop and use temporal and geospatial models, using data from secondary sources, like this project, to estimate the distribution of the malnutrition and enteric infection burden, as well as the benefits attributed to various interventions.
Activity 3 To develop and use temporal and geospatial models, using data from secondary sources, like this project, to estimate the distribution of the malnutrition and enteric infection burden, as well as the benefits attributed to various interventions.
Regarding the INCT-IBISAB, we propose that infection (and co-infection) with certain pathogens can lead to malnutrition, due to the process of intestinal inflammation and/or altering the intestinal barrier and its absorptive function. Thus, we test associations between specific pathogens and malnutrition. Our study aims are to quantify these associations and thus shed light on the following issues: (1) What organisms and mixed infections are more relevant to the deficits in growth and development? (2) At what age in children, such infections cause more specific impact on growth and development?


The Clinical Research Unit (UPC) has its origin attached to the Pharmacology post-graduate course in the UFC and the international research consortium in infectious diseases between the UFC and the University of Virginia, Charlottesville, Va, USA. In 1988, two professors of the Pharmacology post-graduate course felt the need to establish a research core in biomedicine at the Faculty of Medicine, focusing on research lines related to diseases that affect directly the population of northeastern Brazil, such as infectious diseases, malnutrition, diarrheal diseases, hypertension, diabetes, gastritis, peptic ulcers and gastric cancer. UPC develops its research activities based on laboratory quality standards and in compliance with ethical guidelines of national and international standard. After several training sessions and frequent audits performed by national and international foresight such as from the Brazilian Ministry of Health, World Health Organization, the US National Institutes of Health and the Howard Hughes Medical Institute, the UPC acquired in 2009 the concept of global ethics in biomedical research.
Sharing goals and objectives with IBISAB, UPC has worked hard during the biannual of 2009-2010, actively participating in the formation of two international networks, as well as the development and creation of new postgraduate courses, as the newly created DINTER PhD course (Inter-institutional PhD course in Morphological Sciences between UFC and UFRJ), not neglecting the scientific productivity and training of human resources at local, national and international levels. In this period we trained and formed four junior researchers (medical graduate students), a Pharmacology Master Degree student and a post-doc. For details see the website: www.upcibimed.ufc.br.